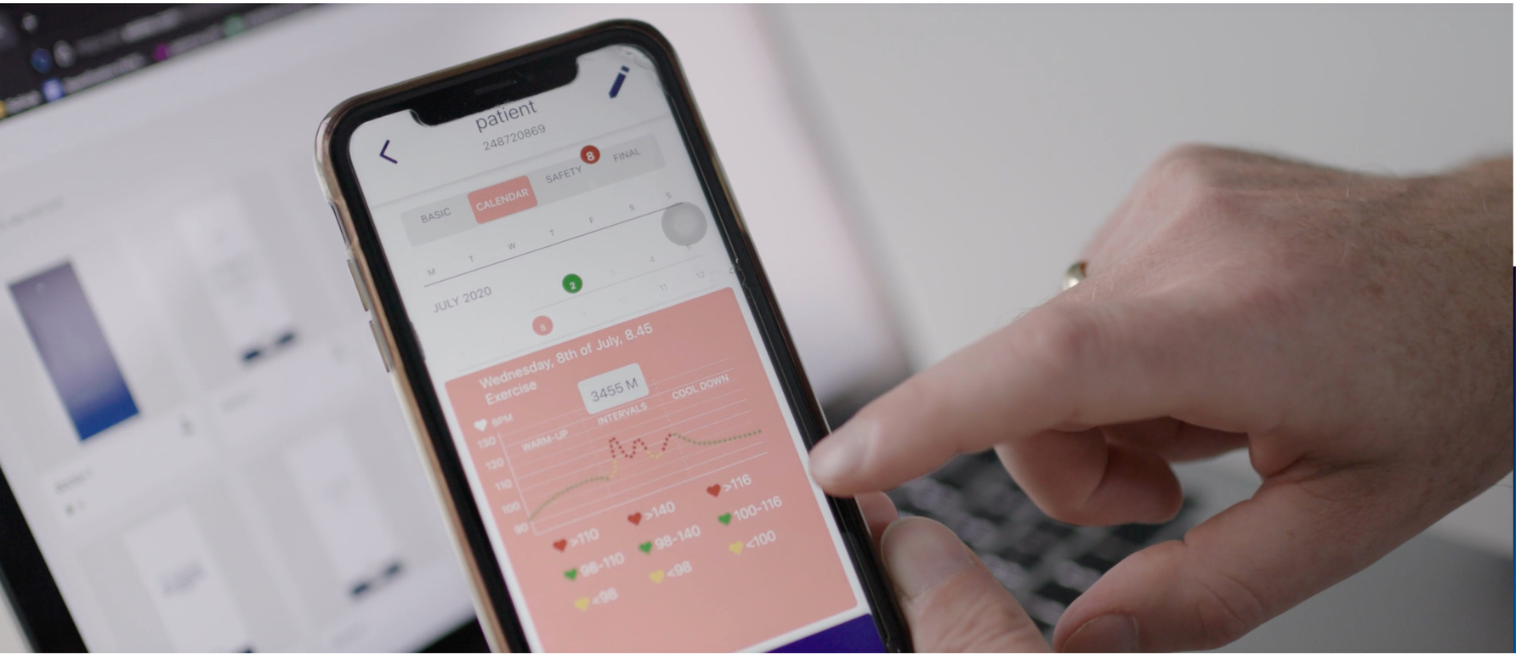
WHO ARE WE?
We are a team of internationally located professionals with quality and innovation-driven approach.
Our team members have extensive knowledge and experience with the healthcare sector, quality of patient care and research. We are creative, proactive and enjoy working together, sharing our company's vision and approach to finding solutions to everyday challenges. Capreolos GmbH was founded with a vision of using digital solutions for improving patient care and experience for both patients and their doctors.
A patient-oriented company
- Our culture and values are focused
on patient safety and quality of care.
- Our approach puts the patient in the centre.
Pioneering
- A revolutionary and visionary app solution that is made for all stakeholders in healthcare.
Medical excellence
- An evidence-based but creative and independant approch in collaboration with patients, doctors and hospitals.